We use cookies
We use cookies to ensure that we give you the best experience on our website. Would you like to accept all cookies for this site?
Baby, Chronic Pododermatitis
Introduction
A five-year-old dog was admitted to the WVS ITC Thailand clinic for treatment of pododermatitis located on the left forepaw. Antibiotic treatment was initiated, with culture and sensitivity required to direct therapy due to the presence of resistance bacteria and the absence of improvement. A total of four courses of antibiotics were used, together with laser therapy and antiseptic foot washes. Finally, the treatment was successful with complete resolution. Read the full story below.
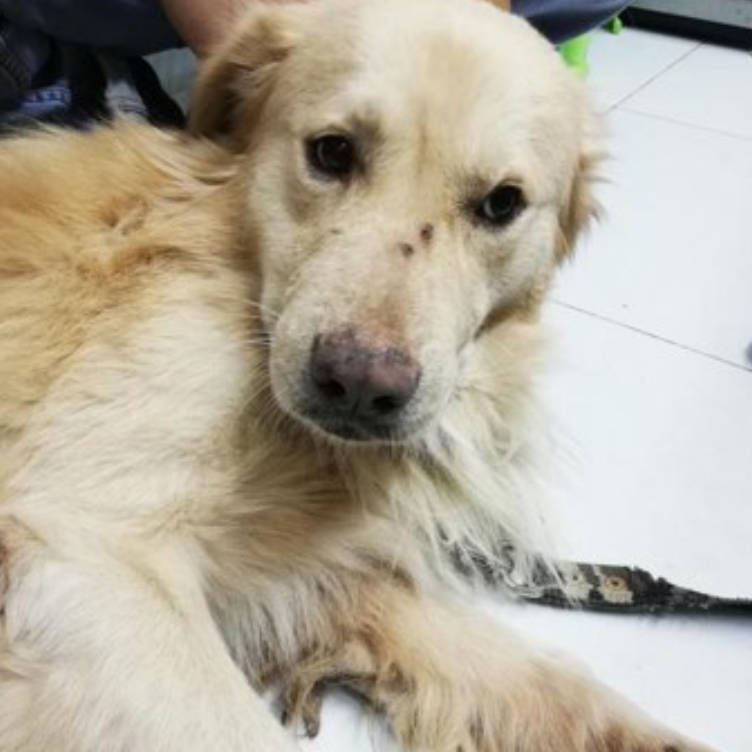
History and Clinical Examination
Baby, a five-year-old intact, male Retriever-cross was presented by the client with an infected wound located on the palmar surface of the left front paw. There was no information about the onset of the wound. Baby's vaccination, ectoparasite and endoparasite treatment was up to date.
The dog was bright, alert and responsive with lameness on his left forelimb. Upon examination, an open wound with ulceration, necrosis, and purulent discharge (pyoderma) was found. The lesion areas included: skin between cranio-medial of metacarpal pad and 2nd digital pad and skin between 2nd and 3rd digital pad, both lesions included the central foot pad (Figure 1). There were no other skin lesions detected on any other part of the body. The remainder of the clinical examination was normal.
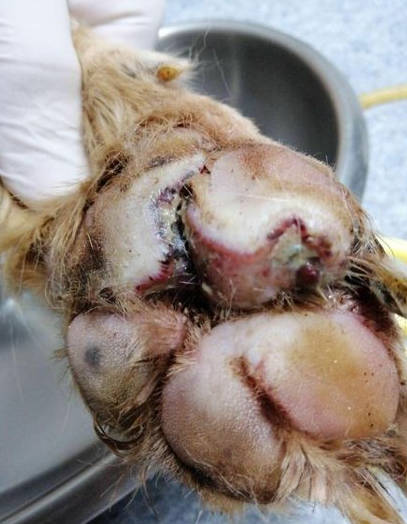
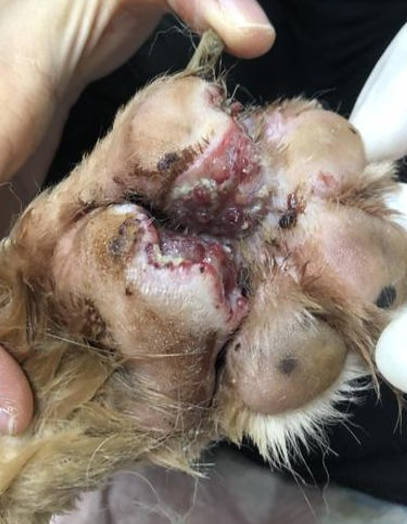
Figure 1 - left image; lesion at day 7 of treatment and picture B; lesion at day 8 of treatment. The pododermatitis lesion presented ulceration, necrotic tissue, and purulent discharge that involved foot pad.
Treatment and Diagnostics
Initial Treatment
Baby was admitted to the WVS Thailand Rescue and Rehabilitation Centre and received per-cutaneous treatment for external parasites. He was started on a course of anitibiotic and anti-inflammatory medications:
- Amoxicillin-clavulanic acid BID (dose 12.9 mg/kg) for 12 days
- Carprofen SID (dose 2.5 mg/kg) for 5 days;
He was fitted with an Elizabethan collar to prevent him from licking the wound and wound dressing was done every day.
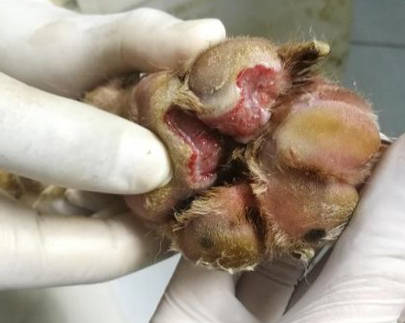
Figure 2 - the wound at day 13 of treatment, after treat with amoxicillin-clavulanate and wound dressing SID. The lesion is ulcerative with inflammation, the purulent discharge and necrotic tissue was decrease.
Bacterial Culture and Sensitivity Results
The first bacterial culture and drug sensitivity was performed after finishing the initial antibiotic treatment and the culture result was Pseudomonas aeruginosa with resistance to amoxicillin-clavulanic acid.
Second Treatment Phase
Antibiotic
The systemic antibiotic was changed to metronidazole SID (dose 25 mg/kg) for 12 days based on the drug sensitivity result.
Analgesia
Baby's left forelimb was banadaged and changed daily. To ensure adequate analgesia, 10mg/kg Gabapentin was administered 2 hours before every wound dressing change and continued for 5 days, and laser therapy was used to encourage healing.
During treatment, due to the painful lesions, general anesthesia was required 4 times (approximately every 14 days) for trimming the hair around the wound and an aggressive wound cleansing and dressing protocol.
The wound seemed to respond to treatment and management. The ulceration of the wound was shallower and smaller, (32 days after the second treatment cycle).
Treatment Set Back
Unfortunately, Baby removed his collar one night, and licking of the wound resulted in re-infection.
Second Bacteria Culture and Sensitivity Results
A second bacterial culture and drug sensitivity was performed, and a growth of Streptococcus-Beta-haemolytic-not group A or B, was reported.
Third Treatment Phase
Antibiotic therapy was changed to clindamycin BID (dose 5 mg/kg) for 21 days combined with enrofloxacin SID (dose 5 mg/kg) for 28 days according to the reported drug sensitivity result.
Care management continued to include a short walk twice a day, with the addition of topical treatment with chlorhexidine/miconazole (Malaseb®1 ml : tap water 30 ml), followed by application of fusidic acid cream.
Third Bacteria Culture and Sensitivity Results
The third bacterial culture and drug sensitivity was performed after the clindamycin course had been given for 11 days, and the result reported Streptococcus-Beta-haemolytic-not group A or B which was resistant to clindamycin but still sensitive to enrofloxacin.
Fourth Treatment Phase
Baby continued to be treated with enrofloxacin plus the Malaseb bathing and fusidic acid topical cream. The wound began to responded well.
Carprofen (dose 2-4 mg/kg) was dispensed intermittently, depending on clinical signs of pain.
By day 78 of the treatment, the wound area had reduced to only the interdigital skin between the 2nd and 3rd digits.
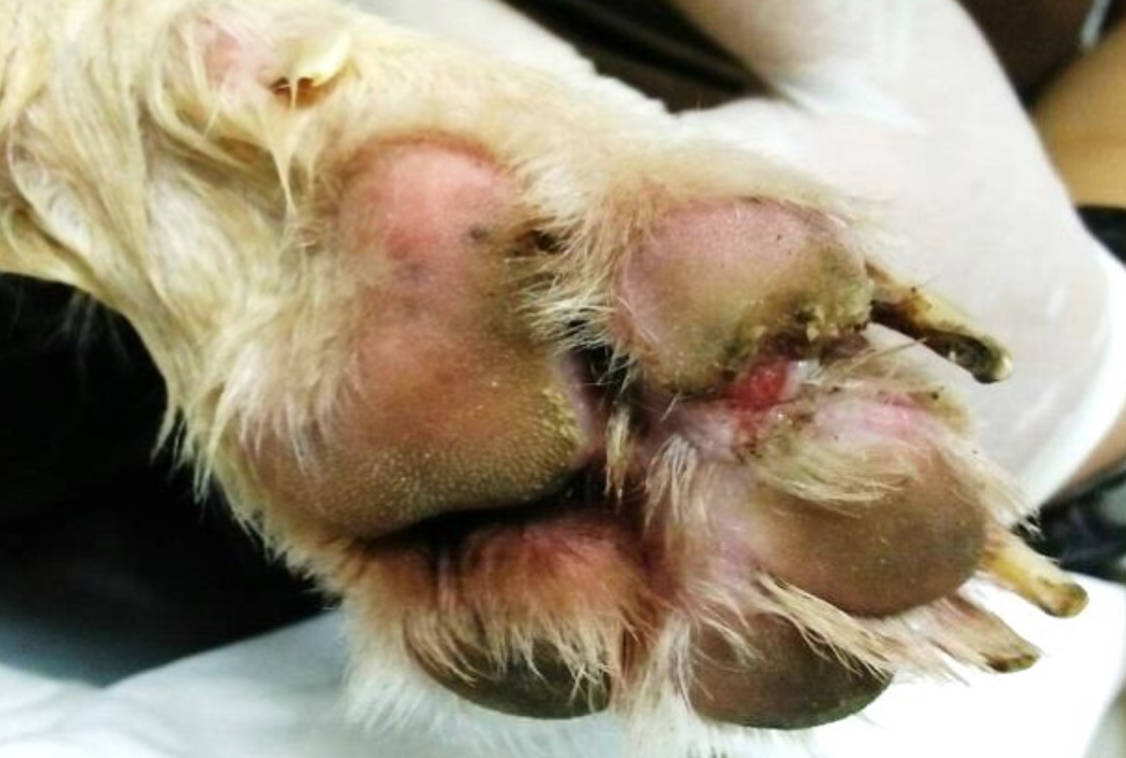
Figure 3 - the wound at day 78 of treatment, after treat with combination of clindaymycin and enrofloxacin for 20 days and foot dipping with Malaseb® and fucidic acid cream for 13 days.
It took a further 20 days of enrofloxacin treatment and malaseb bathing for Baby's wound to have reduced enough for him to be returned to his owner with instructions of bathing every 3 days with 2 weekly check up's at the centre. The treatment course took a total of 98 days from presentation to discharge.
Discussion
Aetiology of pododermatitis
Pododermatitis is inflammation of the skin and connective tissue of the foot. Canine pododermatitis has a wide variety of underlying aetiologies and two of the more common causes are allergic dermatitis with or without a secondary infection, and demodicosis1. There are many causes of pododermatitis and widely differing diseases can present in similar ways. A careful, systematic approach and creation of a list of definitive diagnosis, are essential and demodicosis should always be the first cause to be ruled out in any case of pododermatitis involving haired skin2. In this case, the anti-parasitic spot on given regularly by the owner would control ectoparasites.
Diagnostic procedures
According to the suggested guidelines for using systemic antimicrobials in bacterial skin infections3, cytology is a simple, quick and minimally invasive technique, that can be performed on fully conscious animals with minimum risk and no lasting harm. The diagnosis of pyoderma should be confirmed using cytology prior to the use of systemic antibiotics. Samples for cytology and bacterial culture and sensitivity testing can be taken at the same time.
In this case, we relied on the bacteria culture and sensitivity results and opted for a broad-spectrum antibiotic to begin treatment with, changing this depending on the sensitivity results. However, if a pododermatitis lesion is recurrent, cytology and further diagnosis test (i.e. complete blood count, serum biochemistry, endocrine analysis, skin biopsy, and bacterial culture and drug sensitivity, etc. should be performed1,2.
An example of cytology of skin showing streptococci species from another case is shown below. Bacteria form chains.
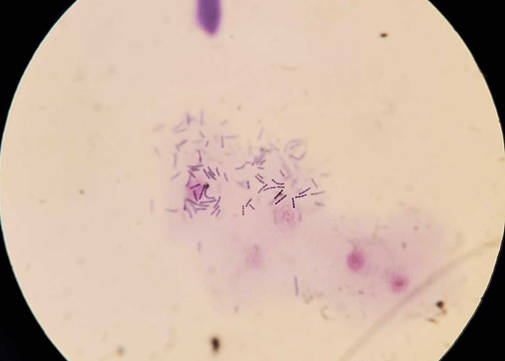
Figure 4 - cytological smear from a skin lesion showing streptococcal bacteria forming short chains (not from this case).
Bacteria Involved
The Streptococci spp found in this case are Gram-positive cocci, facultative anaerobes, catalase-negative, oxidase-negative, and non-motile. They are a pyogenic bacteria that can initiate an infection after injury of the epithelium layer of skin. Streptococcus canis is an opportunistic pathogen found as part of the normal flora of the oropharyngeal, anal and genital mucosa in both dogs and cats, it has antiphagocytic property and cytotoxic substances, the S.canis can be classified into Lancefield group G with Beta haemolysis which can cause skin and wound infections4.
In this case, the second and third bacterial culture results after the dog had removed his collar overnight cultured Streptococcus-Beta-haemolytic-not group A or B. The introduction of this bacteria is likely to have been a direct result of licking the wound.
Antimicrobial use
Systemic
This case had a significant problem with antibiotic resistance. Using the right antibiotics at the correct doses, route of administration and duration is essential to prevent development and propagation of antimicrobial-resistant bacterial strains.
According to the suggested guidelines for using systemic antimicrobials in bacterial skin infections5. The topical treatment with antiseptics and/or antimicrobial shampoo, spray or cream is effective for mild, surface or focal infections. However, if the infection is deep, severe and/or generalised the treatment with systemic antibiotics should be considered. Topical antiseptic treatments can hasten clearing the infection, or will greatly reduce the need for systemic therapy5.
This guideline also classifies antibiotics into first-, second- and third-line antibiotics, where the second-line antibiotics should only be used when there is culture evidence that first-line antibiotics will not be effective. Third-line antibiotics must only be used when there is culture evidence of no sensitivity to the first- or second-line drugs are effective and topical antimicrobial therapy is not feasible or effective.
The duration of treatment depends on the depth of the infection. Superficial pyodermas typically need two to three weeks of treatment and for full resultion of deep pyodermas, four to six weeks or longer is normally required.
In this case, the first-line antibiotic; amoxicillin-clavulanic acid was used for the initial treatment the correct dose was used but it was a short duration; therefore, this could be the result of the resistance seen in the first culture result. After the second and third bacterial culture and drug sensitivity tests, clindamycin and enrofloxacin, which are first- and second-line drugs respectively, had been used in combination for treatment. The correct dose of enrofloxacin was administered, but a slightly lower dose of clindamycin was used according to the suggested guidelines. This could be the reason for resistance seen against clindamycin in the third culture result. Both the enrofloxacin and clindamycin were given for the correct duration.
When managing antibiotic resistant infections in patients, hygiene routines to prevent and control infections and minimise zoonotic risk, should be implemented. These include wearing gloves when in contact with the patient, hand washing before and after wearing gloves, routine disinfection of equipment and surfaces with an antiseptic agent after each case and at the end of the day. Enacting a protocol where patients with known or suspected contagious diseases and infections with multi-resistant bacteria are scheduled to be examined at the end of the day, is also a useful control measure5.
Topical
Fusidic acid is a narrow spectrum fusidane antibiotic which is poorly soluble in water. Indications for the use of this drug are; treatment of otitis externa and skin infections caused by Gram-positive bacteria, particularly surface pyodermas6.
For the final medical treatment plan, the combination of topical treatment by foot dipping with diluted Malaseb® and fusidic acid cream, TID, were effective. As seen with this case, topical antiseptic treatments can hasten resolution of infection and reduce the need for systemic therapy as previously discussed.
Conclusion
In conclusion, canine pododermatitis is a common skin problem but is complicated to treat due to the wide range of causes. Taking a full history and conducting a complete physical examination are very important before initiating treatment. Cytology is a useful diagnostic tool and should be done in every skin disease case.
Skin disease is one of the most common times a clinician will encounter an antimicrobial-resistant bacteria. Therefore, great care should be taken in the selection and use of antibiotics. It is important to refer to the updated antimicrobial guidelines and use bacterial culture and sensitivity results in combination with a care management plan specific to each patient. A combination of treatment techniques, e.g. systemic drugs used, topical drug and antiseptic treatment, laser therapy and hygienic practice are also important for successful treatment.
References
- Christine, R., (2008, October). Differential diagnoses for canine pododermatitis (proceedings). Retrieved from http://veterinarycalendar.dvm360.com/differential-diagnoses-canine-pododermatitis-proceedings, (accessed on 27 Oct. 2019).
- Forsythe, P., (2015, May). Canine Pododermatits [proceedings], WSAVA 2015 Congress. Retrieved from https://www.vin.com/apputil/content/defaultadv1.aspx?pId=14365&catId=73678&id=7259272, (accessed on 27 Oct. 2019).
- Beco, L., Guaguère, E., Méndez, C.L., Noli, C., Nuttall, T., Vroom, M., (2013, January). Suggested guidelines for using systemic antimicrobials in bacterial skin infections (1): diagnosis based on clinical presentation, cytology and culture [open access]. Veterinary Record. 172, 72-78. doi: 10.1136/vr.101069.
- Markey, B., Leonard, F., Archambault, M., Cullinane, A., and Maguire, D., (2013). Chapter 8 the streptococci and related cocci. Clinical veterinary microbiology. (2nd ed.), Edinburgh, Mosby Elsevier, 121-128.
- Beco, L., Guaguère, E., Méndez, C.L., Noli, C., Nuttall, T., Vroom, M., (2013, February). Suggested guidelines for using systemic antimicrobials in bacterial skin infections (2): antimicrobial choice, treatment regimens and compliance [open access]. Veterinary Record. 172, 156-160. doi: 10.1136/vr.101070.
- Vetstream, (n.d.). Fusidic acid. Retrieved from https://www.vetstream.com/treat/canis/generics/fusidic-acid, (accessed on 27 Oct. 2019).
- Plumb, D.C., (2011). Antiseptic - chlorhexidine. Plumb’s veterinary drug book. (7th ed.), Stockholm, Pharma Vet, 3844-3846.
Suggested readings
Frosini, S.M., Bond, R., Loeffler, A., and Larner, J., (2017). Opportunities for topical antimicrobial therapy: permeation of canine skin by fusidic adic [open access]. BMC Veterinary Research. 345(13). doi: 10.1186/s12917-017-1270-6.
Loeffler, A. (2015, September). Time to act on antimicrobial resistance in canine pyoderma. Vet Times. Retrieved from https://www.vettimes.co.uk/article/time-to-act-on-antimicrobial-resistance-in-canine-pyoderma/, (accessed on 26 Oct. 2019).
Tannaz, A., (2002, February). Paw tissues unique; injuries need special care, attention. Retrieved from http://veterinarynews.dvm360.com/paw-tissues-unique-injuries-need-special-care-attention, (accessed on 5 Sep. 2019).

About the author
Dr Malisa Santavakul (aka “Lukpla”) has been working with WVS Thailand since April 2019, after graduating from Kasetsart University in Bangkok and working in the Raptor Centre.
She regularly mentors students (Thai and International) and she looks after Rescue Cases, both surgically and medically.
© WVS Academy 2026 - All rights reserved.