We use cookies
We use cookies to ensure that we give you the best experience on our website. Would you like to accept all cookies for this site?
Tang: Chronic Vomiting and Weight Loss
Summary
The WVS Thailand centre treated a free-roaming dog, with an emaciated appearance and a history of frequent vomiting for several months. Diagnosis proved challenging, although there was some initial improvement through symptomatic treatment. Sadly, the patient’s condition deteriorated, and welfare became a serious concern. The difficult decision was made to euthanise, and post-mortem biopsies revealed an unusual result: infection with Sarcocystis protozoa.

Figure 1 - Tang was treated at our Thailand centre.
History
A 2-year-old, male entire, free-roaming dog was brought to see us, after his community caretaker noticed chronic vomiting for several months, followed by a rapid deterioration in body condition. The caretaker also reported that the dog was constantly salivating and seemed to be frequently swallowing.
Clinical Examination
Findings on clinical examination:
- Body condition score (BCS): 1/5
- Weight: 12.55kg
- Normal hydration, pink mucous membranes, capillary refill time < 2 seconds
- Normal heart and lung sounds
- Abdominal palpation: no pain, organomegaly or mass detected
- All peripheral lymph nodes were normal size.
Initial steps also included taking a blood sample for complete blood count and basic serum profile. A rapid test kit was performed for CPV/CCV, which was negative.
Differential diagnoses
The initial differential diagnoses were:
- Intestinal parasites infection
- Blood parasite infection
- Malnutrition
- Gastritis, enteritis
- GI obstruction, e.g. foreign body
- Inflammatory bowel disease
- Pancreatitis
- Neoplasia
Initial diagnostic tests and treatment
Abdominal radiography and ultrasound were performed the next day. Abdominal radiographs showed a slightly dilated stomach and some normal faeces in the colon. No abnormalities were detected on abdominal ultrasound. Obstruction (e.g. foreign body) and mass therefore seemed less likely.
Blood results showed Hepatozoon canis infection, but other parameters were all within normal range. Infections in dogs are often subclinical, but when clinical signs are present, they may include lethargy, fever and weight loss (1). Treatment for hepatozoonosis was started on his third day with us: doxycycline (dose 10 mg/kg). It was continued until day 12, when it was then stopped to prevent possible side effects (irritation of GI tract). He was dewormed on day eight and day 27 of treatment. Most free-roaming dogs carry a high worm burden, which may have contributed to his clinical signs.
He was also given symptomatic treatment for vomiting:
Ranitidine dose 2 mg/kg) subcutaneously (SC) twice daily (BID) 30 mins before meal (blocks histamine-induced gastric acid secretion, has a prokinetic effect) (2) Metoclopramide (dose 0.5 mg/kg) SC BID 30 mins before meal (anti-emetic, with a very mild prokinetic effect) (2) Sucralfate (Ulcefate) (gastric protectant, which can also stimulate mucosal defences and repair mechanisms) and oral antacid (Antacil Gel) (neutralises gastric acid) both 5 ml PO bid 30 mins before meal (2)
Diet consisted of bland commercial canned food and Royal Canin Recovery formula, with free access to water.

Figure 2 - Tang received ongoing care at our shelter and treatment centre.
The patient was kept in the shelter for treatment, and to monitor vomiting and defecation. Most of the vomitus was saliva and froth, sometimes with partially digested food. No blood (fresh or digested) was noted. Typically, he would vomit three to five times a day, without a clear relation to time of feeding. His general demeanour was bright, active, and responsive. His faeces were formed/solid (faecal score 3/7), and he had a normal appetite. He was happy to be taken for a short walk each day.
Further investigation
The team had no definitive diagnosis and clinical signs persisted. Further investigations were required.
Exploratory laparotomy
On day 12 at the shelter, an exploratory laparotomy was performed, revealing severe gastritis with evidence of bleeding tissue and a slightly enlarged liver and spleen. Intraluminal observation of the intestines and collection of biopsies was not performed due to the severe inflammation in the stomach and risk of causing iatrogenic damage. Castration was performed at the same time as laparotomy for population control.
Repeat radiography and barium study
After receiving ongoing medical treatment for around a month, a decrease in hypersalivation was noted, and frequency of vomiting reduced to once or twice a day.
Repeat plain, abdominal radiography and a barium study were performed on day 30 and 31. Gastric emptying time and intestinal motility appeared to be normal. On day 35 of treatment the dog was sedated to re-examine the larynx and upper oesophageal tract but only mild inflammation (tonsillitis) was found.
Figure 3 - Lateral left barium series (plain film, and 15, 45, 120 mins and 24 hours after patient swallowed barium meal).
Endoscopy
Further investigation was required, so the team fundraised for an endoscopy with a nearby referral service on day 46 of treatment. Bacterial culture and biopsy were performed.
An area of ulceration was found between the pylorus of the stomach and the duodenum. The referral clinician suspected the medical treatment had improved the size/ severity of the lesions. Escherichia coli was cultured from this area, and the biopsy result showed mild catarrhal gastritis.
E.coli has been cultured from healthy dogs, as well as those with gastrointestinal illness (3,4). Whilst there is evidence that some pathogenic strains can cause disease, it can also be part of the normal gut flora in dogs (3,4). In this case, E.coli was thought to be an incidental finding, given the lack of evidence of bacterial infection on the complete blood count, lack of diarrhoea, and the moderate improvement in vomiting without antibiotic therapy. This strain of E.coli had multiple resistance to antibiotics, including all of those available for use in the shelter. There is evidence that multiple-resistant E.coli is a relatively common finding in dogs, and it is important to avoid over-use of antibiotics which may cause contribute to resistance, especially where it is not clinically necessary (5,6).
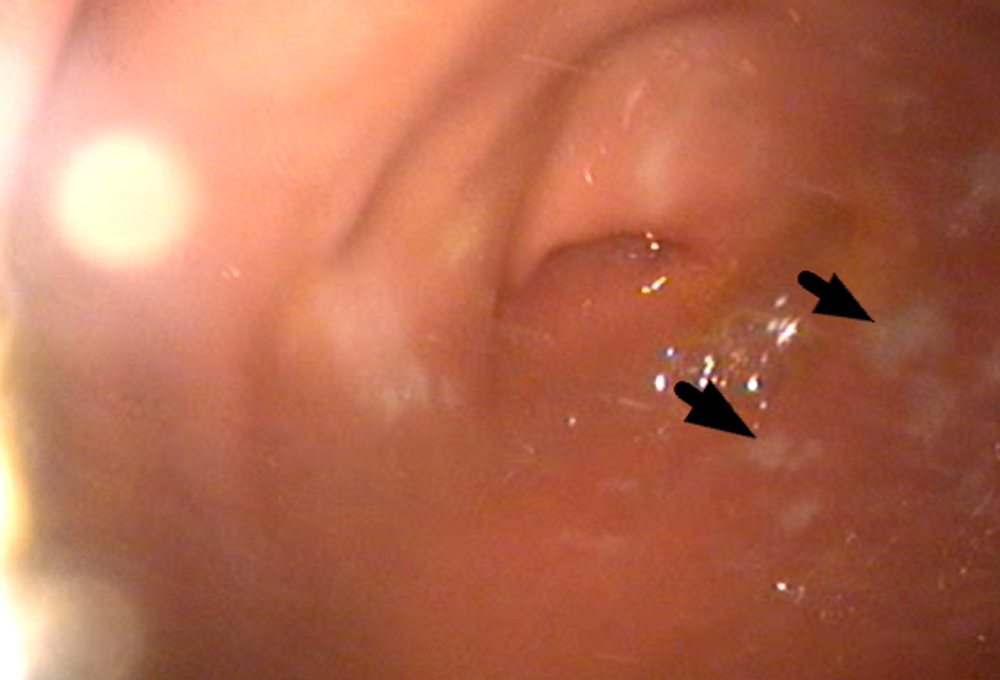
Figure 4 - Endoscopy on day 46 of treatment showed multiple ulcerative lesions (arrows)
Ongoing care and outcome
The primary cause was still unclear, but the clinical signs did start to improve. From day 41 of treatment, there was no further vomiting. He remained on medical treatment, but the dosing regimen was adjusted. Ranitidine and metoclopramide injections were given five minutes before feeding. Sucralfate and oral antacid were mixed with bland food, morning and evening, but he was able to eat bland food with oral medication for his midday meal.
During his time with us at the shelter, seven blood tests were performed. The results were consistently within normal range, except for a very mild increase in ALT (alanine aminotransaminase) on three occasions.
Unfortunately, despite the reduction in vomiting, his BCS slowly worsened (having started very low at 1/5). It was clear that despite medical management, his condition was not improving. The patient’s welfare became a concern. After much consideration, the difficult decision was made to euthanise the patient on welfare grounds.
Post-mortem biopsies were taken from the pancreas, spleen, and the quadriceps muscle. A surprising result was found in the quadriceps muscle: necrosis and myositis, which was characterised as severe, chronic, multifocal to diffuse, with intralesional protozoa infection. The pathologist identified the protozoa as Sarcocystis genus. Further speciation was not performed due to cost.
Discussion
Sarcocystis life cycle and associated clinical signs
Sarcocystosis is caused by a species of protozoal parasites in the Sarcocystis genus. The parasite has an indirect life cycle, and in most cases reproduces sexually in animals such as cats and dogs (the definitive host), and asexually in animals such as ruminants, pigs and horses (the intermediate host).
There are some species of Sarcocystis for which dogs may act as an intermediate host, for instance, S.neurona (7).
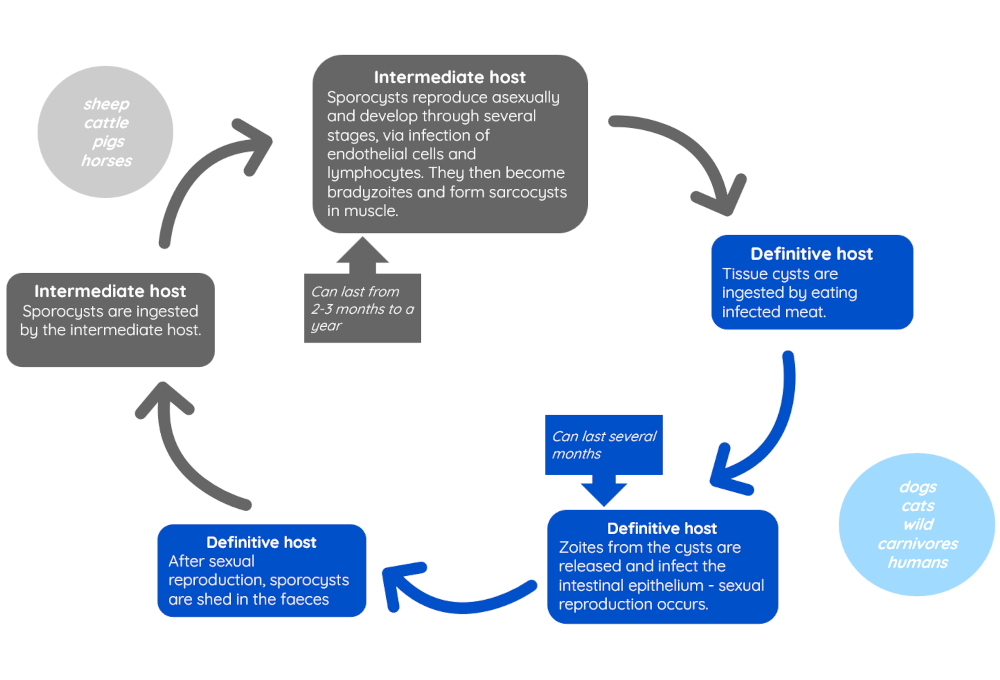
Figure 5 - Life cycle of Sarcocystis (7,9)
In many cases, infection is not of clinical concern and causes few clinical signs. This is especially true in dogs and cats acting as definitive hosts, who generally do not require treatment, although may show self-limiting gastrointestinal signs (7,8). If present, signs usually are associated with stages of the life cycle occurring in the intestines, and affect younger animals most commonly (8). Heavy infestations of intermediate hosts may cause more problems, including in some cases anorexia, fever, weight loss and recumbency (9). In dogs acting as intermediate hosts, myositis and hepatic injury may also occur (10,11).
Other case reports
Sarcocysts have been found in other canine patients, including as incidental findings (12,13). There are relatively few reports of clinical sarcocystosis in dogs, especially associated with myositis, but there are some more recent case reports of interest (10,11).
Chapman et al reported clinical myositis in an adult dog from Canada, associated with an unidentified species of Sarcocystis (10). Granulomatous myositis was associated with numerous immature sarcocysts in a muscle biopsy obtained from the dog. Clinical signs in this case included: fever, dehydration, abdominal pain, ataxia (with occasional petit mal seizure), progressing to an inability to walk, dysphagia, muscle atrophy, generalised pain and weight loss. Blood abnormalities included lymphopenia, low serum total protein, increased alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatinine kinase (CK) and aspartate amino-transferase (AST). Bilirubinuria and haematuria was also noted. After initial treatment with broad spectrum antibiotics, non-steroidal anti-inflammatories and glucocorticoids, the dog was successfully treated with clindamycin for nine weeks, although follow-up was only reported a year after treatment.
Sykes et al reported severe myositis in two dogs as a retrospective case review (11). Initial clinical signs included pyrexia, lethargy, anorexia, and diarrhoea, which progressed to neurological deficits, muscle atrophy and weakness. Blood abnormalities included neutrophilia, monocytosis, anaemia, lymphopenia, thrombocytopenia, increase in liver enzymes and CK, among others. Histopathology of muscle biopsies showed severe inflammatory and necrotizing myopathy with numerous sarcocysts. Both dogs were initially treated unsuccessfully with clindamycin and anti-inflammatory drugs. One dog died 75 days after initial presentation, but the other dog subsequently responded to treatment with decoquinate (follow-up was reported nine months after treatment).
Reflections on this case
Given the biopsy results in this case, it is possible that the patient’s clinical signs were caused by sarcocystosis. The findings of the laparotomy (gastritis, tissue bleeding and mild enlargement of liver and spleen) and the ulceration noted on endoscopy could relate to invasion of gastrointestinal tissue by the parasite. Muscle wastage, weakness and lethargy could be attributed to myositis and formation of sarcocysts in the skeletal tissue. However, none of the blood parameters noted in other reported cases of clinical sarcocystosis were replicated here, except for a mild increase in ALT.
There is limited information available on recommended treatment, but various approaches reported have included antiprotozoal agents (e.g. decoquinate), antibiotics (trimethoprim/ sulfonamide, clindamycin) and steroids (7). Given the rarity of clinical sarcocystosis associated with myositis in dogs, this wasn’t included in the differential diagnoses for this case, and therefore treatment for this condition wasn’t attempted. Methods for diagnosis for intermediate hosts include muscle biopsy (which is not always successful in identifying the parasite, but may have more success post-mortem), and faecal flotation techniques in definitive hosts. Further research is needed on the pathophysiology of sarcocystosis in dogs, and its treatment.
In future cases, sarcocystosis could be considered as a differential for dogs with signs of muscle wastage, weakness, increased liver enzymes and other relevant pathological findings, where alternative explanations have not been identified. This may be particularly relevant to populations of dogs where scavenging on infected meat is more common.
Key points
- Chronic vomiting can be investigated through step-wise diagnostic tests, starting with routine blood tests and abdominal imaging.
- Vomiting symptoms can sometimes be managed or improved using anti-emetic and/or gastroprotectant drugs to improve the welfare of the patient while investigations are carried out.
- It is important to consider the welfare of deteriorating patients whose quality of life becomes compromised despite ongoing treatment and care.
- Sarcocystosis is usually subclinical in dogs and cats, but cases of clinical myositis have been reported and may be considered as a differential in relevant cases.
Author

Dr Malisa Santavakul (aka “Lukpla”) joined WVS Thailand in 2019, after graduating from Kasetsart University in Bangkok, and working in the Raptor Centre. Her interests include mentoring students and looking after rescue cases, both surgically and medically.
References
- Langford Vets, University of Bristol (2021), Hepatazoon species, available at: https://www.langfordvets.co.uk/media/2396/hepatozoon-spp.pdf. [accessed 28/03/2024]
- Allerton,F. (ed.) (2020) Small Animal Formulary(10th edn.) BSAVA: Gloucester
- Hall, E. (2023), Dealing with haemorrhagic diarrhoea in dogs. In Practice, 45: 516-531. https://doi.org/10.1002/inpr.369
- Marks, S.L., Rankin, S.C., Byrne, B.A. and Weese, J.S. (2011), Enteropathogenic Bacteria in Dogs and Cats: Diagnosis, Epidemiology, Treatment, and Control. J Vet Intern Med, 25: 1195-1208. https://doi.org/10.1111/j.1939-1676.2011.00821.x
- Amy L. Wedley, Susan Dawson, Thomas W. Maddox, Karen P. Coyne, Gina L. Pinchbeck, Peter Clegg, Tim Nuttall, Miranda Kirchner, Nicola J. Williams, Carriage of antimicrobial resistant Escherichia coli in dogs: Prevalence, associated risk factors and molecular characteristics, Veterinary Microbiology, Volume 199, 2017, Pages 23-30, ISSN 0378-1135, https://doi.org/10.1016/j.vetmic.2016.11.017.
- Sanchez, S., McCrackin Stevenson, M.A., Hudson, C.R., Maier, M., Buffington, T., Dam, Q. and Maurer, J.J., (2002). Characterization of multidrug-resistant Escherichia coli isolates associated with nosocomial infections in dogs. Journal of Clinical Microbiology, 40(10), pp.3586-3595.
- CSFPH (2020) Sarcocystosis Sarcosporidiosis, Equine Protozoal Myeloencephalitis Pigeon Protozoal Encephalitis. https://www.cfsph.iastate.edu/Factsheets/pdfs/sarcocystosis.pdf Chapman, J., Mense, M., & Dubey, J. P. (2005). Clinical Muscular Sarcocystosis in a Dog. Journal of Parasitology, 91(1), 187–190. https://doi.org/10.1645/ge-406r
- ESCCAP Control of Intestinal Protozoa in Dogs and Cats 06 (2018) (2 edn) Available at: http://www.esccap.org/uploads/docs/5hk9fztt_0701_ESCCAP_Guideline_GL6_v8_1p.pdf. [Accessed 28/3/24]
- Urquhart, G. M., Armour, J., Duncan, J. L., Dunn, A. M., Jennings, F. W., (eds) (1987) Veterinary Parasitology, Longman Scientific & Technical: Harlow
- Chapman J, Mense M, Dubey JP. (2005) Clinical muscular sarcocystosis in a dog. J Parasitol. Feb;91(1):187-90. doi: 10.1645/GE-406R. PMID: 15856899.
- Sykes, J. E., Dubey, J. P., Lindsay, L. L., Prato, P., Lappin, M. R., Guo, L. T., Mizisin, A. P., & Shelton, G. D. (2011). Severe Myositis Associated with Sarcocystis spp. Infection in 2 Dogs. Journal of Veterinary Internal Medicine, 25(6), 1277–1283. https://doi.org/10.1111/j.1939-1676.2011.00828.x
- Hill, J. E., Chapman, W. L., & Prestwood, A. K. (1988). Intramuscular Sarcocystis sp. in Two Cats and a Dog. The Journal of Parasitology, 74(4), 724–727. https://doi.org/10.2307/3282198
- Sahasrabudhe, V.K. and Shah, H.L. (1966), The Occurrence of Sarcocystis sp. in the Dog. The Journal of Protozoology, 13: 531-531. https://doi.org/10.1111/j.1550-7408.1966.tb01956.x
© WVS Academy 2025 - All rights reserved.